drug eluting
Looking ahead, Solaris Endovascular is already enrolling patients in clinical trials for Solaris DE — the first of its kind, drug-eluting covered stent that will address the remaining unmet need in covered stents: edge stenosis.
Solaris DE incorporates sirolimus, a powerful and safe anti-proliferative agent that revolutionized the treatment of coronary artery disease with the virtual elimination of restenosis, from 30% to 3%.
“We expect Solaris DE to deliver similar transformative results to patients with ESKD and PAD.”
-Dr. Marco Costa, Chief Scientific & Medical Officer
Not currently available in the United states
Solaris DE is not currently available.
For more information about Solaris DE please contact us.
Advanced mechanical platform with proven polymer and best in class drug, Sirolimus
Why Sirolimus?
Potent anti-proliferative agent
Inhibits tissue hyperplasia at the edges
Manufacturing know how
20+ years clinical experience
Sirolimus Coating
Biodegradable Polymers
(PLLA/PLGA)
Sirolimus
(Rapamycin)
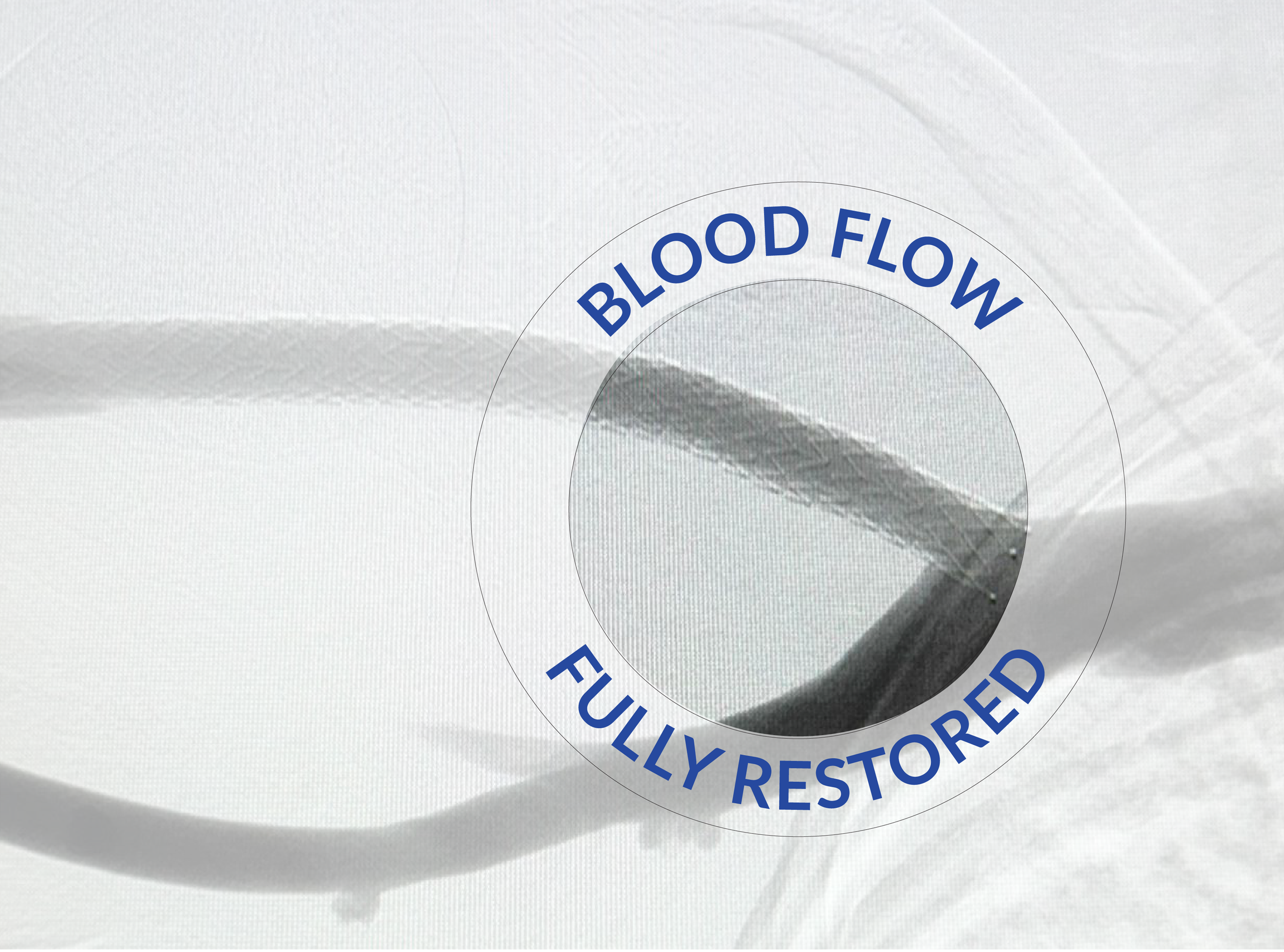
Cephalic Arch Stenosis Treated with Solaris DE
Before treatment with Solaris DE.
After treatment with Solaris DE.
Capturing new market potential with solaris de
Solaris DE long-term patency at 12 months expected to be transformational and to expand the market exponentially, similar to the first drug-eluting coronary stent impact on Percutaneous Coronary Intervention (PCI), moving patients from balloon angioplasty, surgery and medical/catheter management to a more cost-efficient drug-eluting coronary stent.
Solaris DE is expected to deliver similar transformative results to patients with end stage kidney disease (ESKD) and peripheral artery disease (PAD).
Contact Us
Partner with us to redefine patient outcomes for hemodialysis access and PAD.
201 St. Charles Ave, Suite 2500
New Orleans, LA 70170